|
Is a sustainable and economically viable solution 2008
|
♦ Irrigation and salts ♦
It is difficult to manage the salts produced by irrigation, as shown by
current experience in the San Joaquin river and Tulare Lake basins in California.
In order to manage salts properly,
we must first recognize their various types. We must also have a knowledge of their sources, transport paths, and fate.
A salt
is a chemical compound formed by a positively charged ion (cation) and a negatively charged ion (anion).
The salt cations are the following elements: sodium (Na+),
calcium (Ca+), magnesium (Mg+), and potassium (K+).
Typical salt anions are chloride (Cl-), sulfate (SO4-),
and carbonate (CO3-).
The source of all salts is geologic, originating in the parent rocks.
Weathering, and to a smaller extent, the process of bioturbation, disaggregates the rocks and releases
soil particles containing salts in their matrix (inside the particles) and on their surfaces (by adsorption).
Transport by water (surface and subsurface runoff)
releases some of these salts to the water, where they remain in solution until they are used by the biosphere.
Plants are very selective
in their use of salts. Salts of potassium and magnesium are usually in short supply compared to the demand, while salts of sodium and calcium
tend to accumulate in the hydrosphere and lithosphere due to their lack of use by the biosphere.
The selectiveness of the biosphere in its use of salts creates a problem of disposal. What to do with the salts that are not wanted?
For the most part, these salts are wasted, i.e.,
conveyed in solution by the hydrosphere until
a sink of some kind is reached. Nature provides for two distinct salt sinks: (1) the oceans for exorheic drainage systems, and
(2) inland salt lakes for endorheic drainage systems. The ocean precipitates or uses most of the incoming salts, except notably sodium chloride;
therefore, close to 86% of the dissolved salts in ocean waters are sodium chloride.
Inland salt lakes feature a wide range of salts, although sodium chloride
appears to predominate. For instance, close to 60% of the salt ions in the Salton Sea, in California, a repository of agricultural drainage,
are sodium and chloride. Both sodium and chloride
are rejected by most terrestrial flora and fauna.
The biosphere uses water and nutrients, among them, the good salts, i.e., those of potassium and magnesium.
It wastes the bad salts, i.e., those of sodium and calcium. The problem is compounded by the fact that in the use of water by
evapotranspiration, the unwanted salts tend to concentrate in the runoff waters. Thus, headwater streams tend to be low in salt content,
while the lower reaches of rivers tend to be high.
Nature intended for the exorheic basins in the periphery of continents to be flushed of their salts by surface runoff,
thus making them ideally suited to biospheric functioning. She also intended for the endorheic or closed basins in the central regions of continents to
accumulate salts, and thus, to feature low biodiversity. A biblical comparison would refer to the exorheic basins as
near Eden, while the endorheic, salt-afflicted desertic basins would be almost Hell.
Enter Homo Sapiens. Since the start of the agricultural revolution, humans have
learned to grow crops to sustain their sedentary way of life. Eventually, humans learned to do irrigation, that is, to artificially
control the amount of water
used in crop production, thus increasing the productivity and socioeconomic impact of
agriculture. Irrigation is lofty,
but it carries within itself a hindrance: the production of salt in amounts over and above what Nature had intended.
♦ Salt sources ♦
The sources of salts are four: (1) old natural, (2) old artificial, (3) new natural, and (4) new artificial.
Old natural salts are those already present in the soil profile and subsequently leached by the excess irrigation waters.
Old natural salts occur in
fully or partially endorheic geologic settings, such as lakes and inland regions with a geologic history of deficient drainage.
They also occur in near-coastal marine environments that have been subjected to uplift, eventually becoming part of continental areas.
Old artificial salts are those present in the irrigation water, which always has a certain amount of salts. This amount tends to be small
with fresh surface waters (new rain) and large in the case of deep groundwaters. Old artificial salt cannot be avoided.
It is the price to pay for the benefits of irrigation.
New natural salts are by far the most misunderstood. Soils are carriers and producers of salt.
The efficient functioning of the biosphere through irrigation
releases good and bad salts from the soils. The good salts are incorporated into the biomass, while the bad salts are wasted,
together with the old
artificial salts. These salts are typically dissolved in
the waste runoff, eventually flowing into adjacent streams and rivers. A strategy of
reduction or elimination of waste runoff is counterproductive, because it results in the accumulation of salts in the soil
profile, eventually rendering irrigation unprofitable.
New artificial salts are those added by the irrigation system. The good salts are required nutrients. The irrigation system often
fertilizes the land by adding a suitable combination of nutrients, among them, salts of potassium and magnesium. But too much of a good thing
may result in overfertilization and consequent enrichment of the waste runoff. This is commonly called nonpoint-source pollution, to refer
to the fact that an excessive quantity of nutrients was, thorough mismanagement,
allowed to become part of the waste stream and flow into neighboring bodies of water.
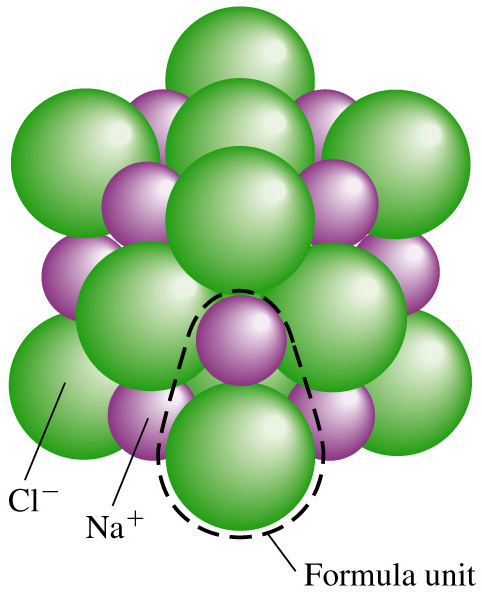
Sodium chloride molecule.

Bioturbation (Carlo Messina, University of Catania, Italy).

Spring saturated with calcium carbonate, Hierve el Agua, Oaxaca, Mexico.

The Salton Sea, in California, a repository of agricultural drainage.

Tufa limestone formations exposed in Mono Lake, California.

Salt-affected irrigation field in the Chao valley, Peru.

The Dead Sea, between Israel and Jordan (photobucket.com).
| Salt sources | Natural | Artificial |
| Old | Already present in the soil profile due to endorheism | Present in the irrigation water |
| New | Present in the soil matrix, and released by weathering and bioturbation | Added by fertilization |
♦ Irrigation engineering ♦
A properly designed irrigation system should minimize old natural salts by carefully choosing the lands to be irrigated to avoid endorheic or partially endorheic environments, and near-coastal areas of marine origin. If this is not possible, a salt budget should indicate the extra amount of old natural salts to be mobilized and properly disposed of. The benefit/cost analysis should include the full cost of proper salt disposal.
The irrigation project should also choose carefully the irrigation waters, to minimize the impact of old artificial salts. For instance, it is poor practice to pump highly saline groundwaters for irrigation and then to accumulate the resulting brackish wastewater in evaporation ponds. This practice is unsustainable and should be discouraged.
The new natural salts cannot be avoided. It is the price to pay for the benefits afforded by the additional amount of food and fiber realized by irrigation. Still, a carefully prepared salt budget should indicate the extra amount of new natural salts to be mobilized and properly disposed of. As with the old natural salts, the benefit/cost analysis should include the full cost of proper salt disposal.
 |
| Parallel irrigation and drainage canals, Wellton-Mohawk, Arizona. |
The new artificial salts can be avoided by proper on-farm fertilizer management. Failure to carry out this management will result in the eventual eutrophication of adjacent water bodies. This practice is unsustainable and should be discouraged.
♦ Tragedy of the Commons ♦
The contemporary issues of irrigation salt management are seen to be very complex and costly. The question is: Is a sustainable solution, which also makes economic sense, possible? At this juncture, we urgently need a holistic view of science and technology, i.e., the meshing of geology, geomorphology, hydrology, ecology, agriculture, economics, sociology, and political science into a coherent, workable entity. In the case of salt, it appears that the only sustainable solution, however wasteful in appearance, is to allow the rivers to reassert their natural function of carrying the salts to the ocean, where they can remain, forever, out of sight and out of mind. This entails reserving a certain fraction of the river runoff for this intrinsic natural purpose (Pillsbury, 1981). In other words, every basin must have a limit imposed on the anthropogenic conversion of runoff to evapotranspiration through irrigation. Such a limit is seen to be absolutely necessary to avoid a repetition of the "Tragedy of the Commons" (Hardin, 1968).
 |
