| Design and Performance of Waste Stabilization Ponds | |||||
| Hamzeh Ramadan and Victor M. Ponce
Version 050203 | |||||
|

2. Waste Stabilization Ponds Systems 3. Waste Stabilization Ponds Types and Functions 4. Additional Technologies Used to Improve WSP | |||||
|
1. Introduction
The most appropriate wastewater treatment
is that which will produce an effluent meeting the recommended microbiological and chemical quality guidelines both at
low cost and with minimal operational and maintenance requirements (Arar, 1988). Adopting as low a level of treatment as possible is
especially desirable in developing countries, not only from the point of view of cost but also in acknowledgement of the difficulty
of operating complex systems reliably. In many locations it will be better to design the reuse system to accept a low-grade of effluent
rather than to rely on advanced treatment processes producing a reclaimed effluent which continuously meets a stringent quality standard.
Waste Stabilization Ponds (WSP) are now regarded as the method of first choice for the treatment of wastewater in many parts
of the world. In Europe, for example, WSP are very widely used for small rural communities (approximately up to 2000 population but larger systems
exist in Mediterranean France, and also in Spain and Portugal) (Boutin et al., 1987; Bucksteeg, 1987). In the United States one third of all
wastewater treatment plants are WSP, usually serving populations up to 5000 (EPA, 1983). However in warmer climates (the Middle East, Africa,
Asia and Latin America) ponds are commonly used for large populations (up to around 1 million). In developing countries and especially in the
tropical and equatorial regions sewage treatment by WSPs has been considered an ideal way of using natural processes to improve sewage effluents.
Waste Stabilization Ponds (WSP), often referred to as oxidation ponds or lagoons, are holding basins used for secondary
wastewater (sewage effluents) treatment where decomposition of organic matter is processed naturally, i.e. biologically. The activity in the
WSP is a complex symbiosis of bacteria and algae, which stabilizes the waste and reduces pathogens. The result of this biological process is to convert the
organic content of the effluent to more stable and less offensive forms. WSP are used to treat a variety of wastewaters, from domestics
wastewaters to complex industrial waters, and they function under a wide range of weather conditions, i.e. tropical to arctic.
They can be used alone or in combination with treatment processes.
A WSP is a relatively shallow body of wastewater contained in an earthen man-made basin into which wastewater
flows and from which, after certain retention time (time which takes the effluent to flow from the inlet to the outlet) a well-treated effluent
is discharged. Many characteristics make WSP substantially different from other wastewater treatment. This includes design,
construction and operation simplicity, cost effectiveness, low maintenance requirements, low energy requirements, easily adaptive
for upgrading and high efficiency.
2. Waste Stabilization Ponds Systems A World Bank Report (Shuval et al. 1986) endorsed the concept of stabilization pond as the
most suitable wastewater treatment system for effluent use in agriculture. Table 1 provides a comparison of the advantages and
disadvantages of ponds with those of high-rate and low-rate biological wastewater treatment processes (note that Aereated Lagoon
and WSP system are considered low-rate biological wastewater treatment processes). Stabilization ponds are the preferred wastewater
treatment process in developing countries, where land is often available at reasonable opportunity cost and skilled labor is in short supply. Table 1. Advantages and disadvantages of various sewage treatment
systems (Arthur 1983). Criteria Package plant Activated sludge plant Extended aeration activated sludge Biological filter Oxidation ditch Aerated lagoon Waste stabilization pond system Plant performance BOD removal F F F F G G G FC removal P P F P F G G SS removal F G G G G F F Helminth removal P F P P F F G Virus removal P F P P F G G Economic factors Simple and cheap construction P P P P F F G Simple operation P P P F F P G Land requirement G G G G G F P Maintenance costs P P P F P P G Energy demand P P P F P P G Sludge removal costs P F F F P F G Wastewater stabilization pond systems are designed to achieve different forms of treatment in
up to three stages in series, depending on the organic strength of the input waste and the effluent quality objectives.
For ease of maintenance and flexibility of operation, at least two trains of ponds in parallel are incorporated in any design.
Strong wastewaters, with BOD5 concentration in excess of about 300 mg/l, will frequently be introduced into first-stage
anaerobic ponds, which achieve a high volumetric rate of removal. Weaker wastes or, where anaerobic ponds are environmentally unacceptable, even stronger wastes
(say up to 1000 mg/l BOD5) may be discharged directly into primary facultative ponds. Effluent from first-stage
anaerobic ponds will overflow into secondary facultative ponds, which comprise the second-stage of biological treatment. Following
primary or secondary facultative ponds, if further pathogen reduction is necessary, maturation ponds will be introduced to
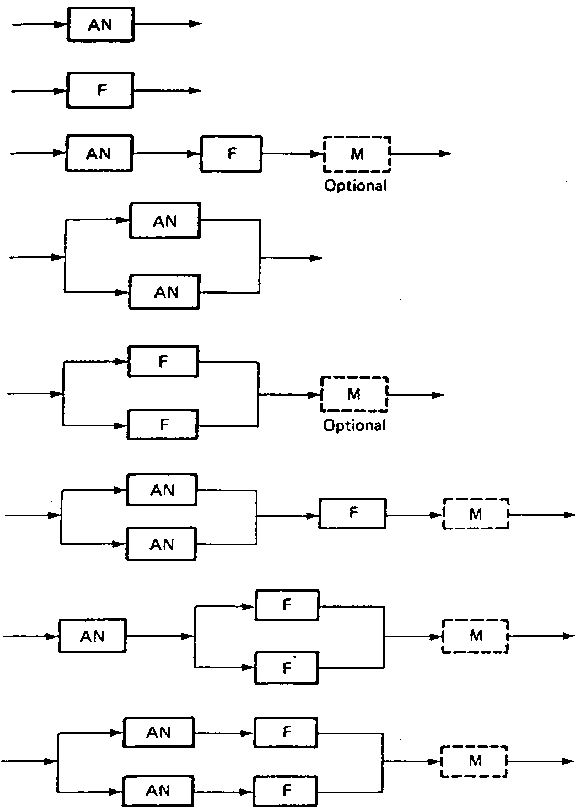
provide tertiary treatment. Typical pond system configurations are given in Fig. 1, though other combinations may be used. Fig. 1 Stabilization pond configurations: AN = anaerobic pond; F = facultative pond;
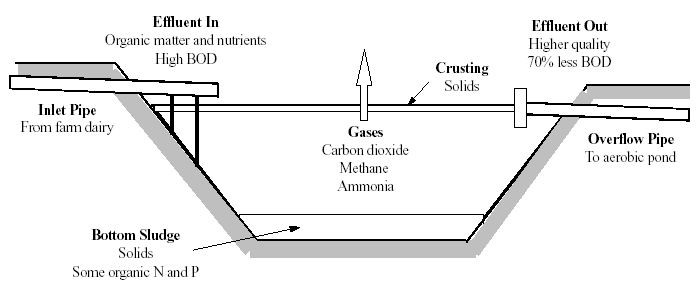
WSP can be classified in respect to the type(s) of biological activity occurring in a pond. Three types are distinguished: anaerobic, facultative and maturation ponds. Usually a WSP system comprises a single series of the aforementioned three ponds types or several such series in parallel (see Section 2). In essence, anaerobic and facultative ponds are designed for BOD removal (Biological Oxidation Demand-see Section 3.1.1) and maturation ponds for pathogen removal, although some BOD removal occurs in maturation ponds and some pathogen removal in anaerobic and facultative ponds. In many instances only anaerobic and facultative ponds are required. In general, maturation ponds are required only when stronger wastewaters (BOD > 150 mg/l) are to be treated prior to surface water discharge and when the treated wastewater is to be used for unrestricted irrigation (irrigation for vegetable crops). Generally, in WSP systems, effluent flows from the anaerobic pond to the facultative pond and finally, if necessary, to the maturation pond. However, for better results wastewater flowing into an anaerobic pond shall be preliminary treated in order to remove coarse solids and other large materials often found in raw wastewater. Preliminary treatment operations typically include coarse screening, grit removal and, in some cases, comminution of large objects. [Top]3.1. Anaerobic Ponds Anaerobic ponds are deep treatment ponds that exclude oxygen and encourage the growth of bacteria, which break down the effluent. It is in the anaerobic pond that the effluent begins breaking down in the absence of oxygen "anaerobically". The anaerobic pond acts like an uncovered septic tank. Anaerobic bacteria break down the organic matter in the effluent, releasing methane and carbon dioxide. Sludge is deposited on the bottom and a crust forms on the surface as shown in Fig. 2.
Fig. 2 Operation of the Anaerobic Pond. Anaerobic ponds are commonly 2-5 m deep and receive such a high organic loading (usually > 100 g BOD/m3 d equivalent to > 3000 kg/ha/d for a depth of 3 m). They contain an organic loading that is very high relative to the amount of oxygen entering the pond, which maintains anaerobic conditions to the pond surface. Anaerobic ponds don't contain algae, although occasionally a thin film of mainly Chlamydomonas can be seen at the surface. They work extremely well in warm climate (can attain 60-85% BOD removal) and have relatively short retention time (for BOD of up to 300 mg/l, one day is sufficient at temperature > 20oC). Anaerobic ponds reduce N, P, K and pathogenic microorganisms by sludge formation and the release of ammonia into the air. As a complete process, the anaerobic pond serves to:
These fermentation processes and the activity of anaerobic oxidation throughout the pond remove about 70% of the BOD5 of the effluent. This is a very cost-effective method of reducing BOD5. Normally, a single anaerobic pond in each treatment train is sufficient if the strength of the influent wastewater is less than 1000 mg/l BOD5. For high strength industrial wastes, up to three anaerobic ponds in series might be justifiable but the retention time in any of these ponds should not be less than 1 day (McGarry and Pescod, 1970). Designers have been in the past too afraid to incorporate anaerobic ponds in case they cause odor. Formation of odor is strongly dependent on the type of waste to be treated in the plant, notably its sulphate (SO4) concentration and volumetric loading rate, respectively. SO4 is reduced to hydrogen sulphide (H2S) under anaerobic conditions. H2S is the compound mainly responsible for obnoxious odors. Other components besides H2S and originating from the anaerobic decomposition of carbohydrates and proteins may contribute to obnoxious odors, too. However, odor is not a problem if the recommended design loadings are not exceeded and if the sulphate concentration in the raw wastewater is less than 300 mg SO4/l (Gloyna and Espino, 1969). A small amount of sulphide is beneficial as it reacts with heavy metals to form insoluble metal sulphides, which precipitate out. In the case of typical municipal sewage, it is generally accepted that a maximum anaerobic pond loading of 300 g BOD5/m3 d at 200C will prevent odor nuisance (Mara et al. 1992). However, results obtained from a more recent study in northern Brazil carried out by Pearson et al. (1996) suggest that maximum design volumetric loadings may increase to 350 g BOD5/m3d at 25°C rather that restricting it to 300 g BOD5/m3d at 20°C. Furthermore, Mara and Pearson (1986) propose a maximum sulphate volumetric loading rate of 500 g SO4/m3 d (equivalent to 170 g S/ m3d) in order to avoid odor nuisance. [Top]3.1.1. BOD Removal Rates and Factors First, the concept of Biological Oxidation Demand (BOD) should be introduced. Organic compounds in wastewater may be used as food for bacteria, which can biochemically digest or oxidize the organic compounds to produce energy for growth. This oxidation of organic material, if done under aerobic conditions (i.e. in the presence of oxygen), "consumes" oxygen and produces carbon dioxide. An organic waste can therefore be said to have a biochemical oxygen demand, i.e. the amount of oxygen required by aerobic bacteria to oxidize it. The term BOD is used to refer to the organic material in a waste and can be used in quantitative expressions relating to organic material, i.e. the expression g BOD or kg BOD describes an amount of organic material. The amount of BOD in a specific volume of wastewater is the concentration or strength of the wastewater and is expressed in terms such as g/m3 or mg/L or parts per million of BOD (all numerically equivalent). The loading rate of organic waste to a treatment system or a receiving environment (i.e. land) is expressed as a mass of BOD/volume (or area) of treatment system per unit of time: i.e., g BOD/m3/day for loading rate of an anaerobic pond; g BOD/m2/day to a facultative pond or to land. BOD is measured in a five-day test of oxygen consumption. The BOD value derived from this test is usually expressed as the BOD5 of the wastewater. Small ponds that receive a reasonably high input of plant nutrients generally develop ecosystems that feature algal populations that produce oxygen in excess of the respiration requirements of the algae. This "excess" oxygen can be used by bacteria to oxidize biodegradable organic matter (quantified as BOD5) entering the pond. This principle forms the basis of natural-aeration waste stabilization ponds, wherein bacterial degradation of organic waste provides carbon dioxide and nutrients to sustain algal photosynthesis and production of oxygen that the bacteria then use. In anaerobic ponds BOD removal is achieved (as in septic tanks) by sedimentation of settleable solids and subsequent anaerobic digestion in the resulting sludge layer: this is particularly intense at temperatures above 15oC when the pond surface literally bubbles with the release of biogas (around 70 percent methane and 30 percent carbon dioxide); methane production increases sevenfold for every 5oC rise in temperature (Marais, 1970). The biochemical reactions that take place in anaerobic ponds are the same as those occurring in anaerobic digesters, with a first phase of acidogenesis and a second slower-rate of methanogenesis. Ambient temperatures in hot-climate countries are conducive to these anaerobic reactions and expected BOD5 removals for different retention times in treating sewage have been given by Mara (1976) as shown in Table 2. More recently, Gambrill et al. (1986) have suggested conservative removals of BOD5 in anaerobic ponds as 40% below 10°C, at a design loading of 100 g/m3d, and 60% above 20°C, at a design loading of 300 g/m3d, with linear interpolation for operating temperature between 10 and 20°C. Higher removal rates are possible with industrial wastes, particularly those containing significant quantities of organic settleable solids. Of course, other environmental conditions in the ponds, particularly pH, must be suitable for the anaerobic microorganisms bringing about the breakdown of BOD. Table 2. BOD removals in Anaerobic Ponds
loaded
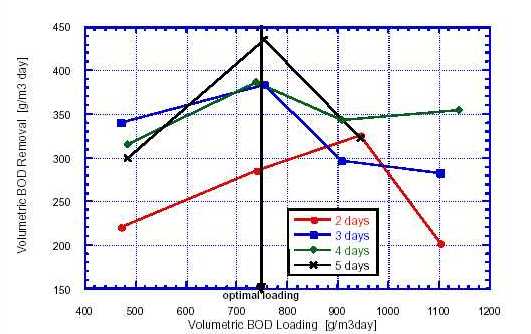
Anaerobic ponds are normally designed on the basis of a temperature-dependent empirical value for the permissible organic loading rate. Land requirements will be lowest if the maximum possible BOD loading can be applied. The upper limit of the volumetric BOD loading is determined by odor emissions and minimum pH threshold value at which the anaerobic decomposition processes cease to work. The maximum BOD loading rate acceptable to avoid odor nuisance was discussed earlier in section 3.1. However, the effect of pH must be taken into consideration. Concentrations of H2S, which is the sulphur form responsible for odors, increases sharply as the pH drops below 7.5, phenomenon which may occur if an anaerobic pond is heavily loaded or overloaded (based on a BOD loading rate criterion). Sulphide may also impede methane production in anaerobic ponds if occurring at excess concentrations. The presence of heavy metals will lead to insolubilisation of sulphides (e.g. iron sulphides). Since methanogenesis is the rate-limiting factor in anaerobic metabolism, products from the preceding acidogenesis reaction may accumulate and lead to a pH decrease. Optimum pH for methanogenesis amounts to 6.0 - 8.0. Based on various anaerobic digestion studies, McGarry and Pescod (1970) found that pH = 6.0 probably constitutes the lowest limit for anaerobic tropical ponds. Acidic wastewaters thus require neutralizing prior to treatment in anaerobic ponds as a low pH can be considered a toxicant for anaerobic bacteria. Determination of the maximum BOD loading rate beyond which pH is likely to drop below this threshold value is, therefore, important. A study on anaerobic pond treatment of tapioca starch waste conducted by Uddin (1970) revealed that a volumetric BOD loading rate of around 750 g/m3·d resulted in a pond pH of 6.0. Fig. 3, which is based on Uddin's results shows that when the BOD loading rate was increased above this value, the volumetric BOD removal rate was reduced. Most likely, pond overloading impaired methanogenesis.
Fig. 3 Influence of Retention Time and Volumetric BOD Loading Rate on Volumetric BOD The published BOD elimination rates for anaerobic wastewater ponds range from 50 to 85%. Temperature, retention time and BOD loading rate affect removal efficiency. Furthermore, the type of substrate; i.e., sewage, septage or public toilet sludge and its concentration influence the physical and biochemical processes. To achieve high elimination rates at the start of a new operating cycle, some sludge should be left for seeding when emptying a pond. Experience with anaerobic pond treatment in tropical climate reveals that anaerobic digestion is basically completed after about four days (van Haandel and Lettinga 1994). Highest BOD elimination and, thus, reduction of land requirements are attained by applying the highest permissible BOD loading rate (loading limits were discussed before). Multi-stage anaerobic ponds, each operated at a maximum BOD loading rate, will, therefore, have the lowest land requirements. If the influent is of high strength (BOD > 8,000 and COD = 20,000-50,000 mg/l), such as public toilet sludge without co-mixture of septage, removal rates (expressed in g/m3·d) will be higher in a multi-stage pond than in a single anaerobic pond. When treating wastewater of low strength (BOD < 2,000 and COD < 10,000 mg/l), high BOD pond loading rates will lead to very short retention times. This may, in turn, cause a decrease in the BOD removal rate. Fig. 3, derived from data presented by McGarry and Pescod (1970) on work performed by Uddin (1970), shows that the BOD removal rates for tapioca starch waste decrease at decreasing retention times, and increase to a threshold value if BOD loading rates are increased. Another factor may affect the BOD and COD removal, which is the ammonia (NH3) toxicity to anaerobic bacteria. Experiments conducted by Sergrist (1997) showed a 50% growth inhibition at a NH3-N/l concentration of 25-30 mg/l. Strong ammonia inhibition in anaerobic ponds can occur at concentrations >80 mg NH3-N/l and may reduce significantly COD elimination to as low as 10% in primary anaerobic ponds (Data is still scarce in this matter). In certain instances, anaerobic ponds become covered with a thick scum layer, which is thought to be beneficial but not essential, and may give rise to increased fly breeding. Solids in the raw wastewater, as well as biomass produced, will settle out in first-stage anaerobic ponds and it is common to remove sludge when it has reached half depth in the pond. This usually occurs after two years of operation at design flow in the case of municipal sewage treatment. [Top]3.1.2. Pathogen Removal In natural treatment systems such as WSP, the pathogens are progressively removed along the ponds series with the highest removal efficiency taking place in the maturation ponds (Mara et al., 1992). However, the following observations can be carried out from different studies that discussed anaerobic ponds participation in pathogen removal:
In WSP systems the nitrogen cycle is at work, with the probable exception of nitrification and denitrication. In anaerobic ponds organic nitrogen is hydrolyzed to ammonia, so ammonia concentrations in anaerobic pond effluents are generally higher than in the raw wastewater (unless the time of travel in the sewer is so long that all the urea has been converted before reaching the WSP). Volatilization of ammonia seems to be the only likely nitrogen removal mechanism occurring to some extent in anaerobic ponds. Soares et al (1996) carried found a very low removal of nitrogen in anaerobic ponds. Phosphorus The mechanisms of phosphorus removal most likely take place in maturation ponds (Mara et al. 1992). 3.1.4. Environmental Considerations Physical as well as chemical factors affect the habitat of microorganisms and consequently the anaerobic sewage treatment process. The most important environmental factors to take into consideration are: temperature, pH, degree of mixing, nutrient requirements, ammonia and sulphide control and the presence of toxic compounds in the influent (Van Haandel and Lettinga, 1994). Temperature As temperature rises, the rate of reaction also increases. In order to have a reasonable methane production rate, the temperature should be maintained above 20°C. Methane production rates are doubled for each 10°C temperature increase in the mesophilic range (Droste, 1997). pH According to Zehnder et al. (1982), the optimum pH range for all methanogenic bacteria is between 6 and 8, but the optimum value for the group as a whole is close to 7. Van Haandel and Lettinga (1994) reported the same observation and also pointed out that, since acidogenic populations are notably less sensitive to pH variations, acid fermentation will predominate over methanogenic fermentation. The latter may result in souring of the reactor contents. Thus, the system must contain adequate buffering capacity to neutralize the production of volatile acids and carbon dioxide, which dissolves at the operating pressure (Droste, 1997). Degree of Mixing The separation of digestion from other processes and the application of mixing were the first major advances in anaerobic treatment. Mixing is an important factor in pH control and maintenance of even environmental conditions. It distributes buffering agents throughout the reactor volume and prevents localized build-up of high concentrations of intermediate metabolic products, which may inhibit methanogenic activity. On the contrary, inadequate mixing propitiates the development of adverse microenvironments. Nutrient Requirements Acidogenic and methanogenic bacteria have low growth rates for a given amount of substrate and this feature results in less nutrient requirements compared to aerobic systems. On the other hand, anaerobic systems produce 20% or less of the amount of sludge produced in aerobic systems for the same substrate and so N and P requirements should decrease proportionally. Ammonia and Sulphide Control Anaerobic bacteria can acclimatize to high ammonia concentrations, but large fluctuations can be detrimental to the process. Free ammonia is much more toxic than the ammonium ion and it occurs more at high pH values. Wastes with high contents of proteins will generate significant amounts of ammonia that in turn increases alkalinity. Wastes containing blood can produce enough ammonium bicarbonate to raise the pH beyond the optimal range and this requires acid addition for pH correction. In most cases, the protein content of wastes is not high enough to cause ammonia toxicity problems. At the same time, sulphide can be formed in the process due to the reduction of sulphates. Sulphides are inhibitory to methanogens and sulphate-reducers themselves, but according to results of Rinzema (1988), a sulphide concentration of up to 50 mg/l (normally expected in anaerobic sewage treatment systems) is far lower than the minimum concentration causing toxicity problems. Toxic Compounds Other compounds such as heavy metals and chloro-organics affect the rate of anaerobic digestion even at very low concentrations. Apart from sulphide, oxygen is also a potentially toxic compound, which can enter the reactor together with influent flow. However, the presence of these compounds at inhibitory concentrations is unlikely in domestic wastewater.
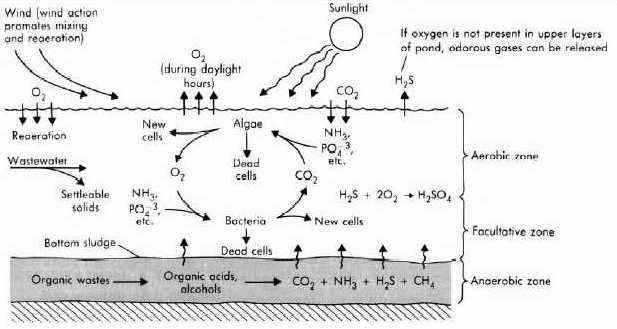
3.2. Facultative Ponds Facultative ponds (1-2 m deep) are of two types: primary facultative ponds, which receive raw wastewater, and secondary facultative ponds, which receive settled wastewater (usually the effluent from anaerobic ponds). They are designed for BOD removal on the basis of a relatively low surface loading (100-400 kg BOD/ha d at temperature between 20°C and 25°C) to permit the development of a healthy algal population as the oxygen for BOD removal by the pond bacteria is mostly generated by algal photosynthesis. Due to the algae facultative ponds are colored dark green, although they may occasionally appear red or pink (especially when slightly overloaded) due to the presence of anaerobic purple sulphide-oxidizing photosynthetic bacteria. The algae that tend to predominate in the turbid waters of facultative ponds are the motile genera (such as Chlamydomonas, Pyrobotrys and Euglena) as these can optimize their vertical position in the pond water column in relation to incident light intensity and temperature more easily than non-motile forms (such as Chlorella, although this is also fairly common in facultative ponds). The concentration of algae in a healthy facultative pond depends on loading and temperature, but is usually in the range 500-2000 µg chlorophyll a per litre. How Facultative Ponds Work? Effluent entering the facultative pond from the anaerobic pond (secondary facultative pond) is converted into carbon dioxide, water and new bacterial and algae cells in the presence of oxygen, i.e., aerobically. Algae populations within the aerobic pond require sunlight. They develop and produce oxygen in excess of their own requirements. It is this excess of oxygen that is used by bacteria to further break down the organic matter within the effluent. The algal production of oxygen occurs near the surface of aerobic ponds to the depth to which light can penetrate (i.e. typically up to 500 mm). Oxygen can also be introduced by wind. Aerobic pond is more accurately termed "facultative", as in practice the pond usually has an aerobic upper layer and anaerobic lower layer. This facultative condition occurs because high oxygen levels cannot be maintained to the total depth of aerobic ponds. So a fully aerobic surface layer develops, along with an aerobic/anaerobic intermediate layer, and a fully anaerobic layer on the pond bottom. Oxygen is unable to be maintained at the lower layers if:
As a result of the photosynthetic activities of the pond algae, there is a diurnal variation in the concentration of dissolved oxygen. For a typical facultative pond, the water column will be predominantly aerobic at the time of peak sun radiation and predominantly anaerobic at sunrise. After sunrise, the dissolved oxygen level gradually rises to a maximum in the mid-afternoon, after which it falls to a minimum during the night. The position of the oxypause (the depth at which the dissolved oxygen concentration reaches zero) similarly changes, as does the pH since at peak algal activity carbonate and bicarbonate ions react to provide more carbon dioxide for the algae, so leaving an excess of hydroxyl ions with the result that the pH can rise to above 9 which kills faecal bacteria. The wind has an important effect on the behavior of facultative ponds, as it induces vertical mixing of the pond liquid. Good mixing ensures a more uniform distribution of BOD, dissolved oxygen, bacteria and algae and hence a better degree of waste stabilization. In the absence of wind-induced mixing, the algal population tends to stratify in a narrow band, some 20cm thick, during daylight hours. This concentrated band of algae moves up and down through the top 50 cm of the pond in response to changes in incident light intensity, and causes large fluctuations in effluent quality (especially BOD and suspended solids) if the effluent take-off point is within this zone. The operation of the facultative pond is shown in Fig. 4.
Fig. 4 Operation of the facultative pond (Tchobanoglous and Schroeder 1987). The facultative pond will remove odor and kill most pathogenic microorganisms. As a complete process, the facultative pond serves to:
Sometimes two or more consecutive smaller facultative ponds are constructed instead of a very large one. This may be more practical for effective desludging and stirring or when the pond is too long for the site and interferes with existing structures. In primary facultative ponds (those that receive raw wastewater) the above functions of anaerobic and secondary facultative ponds are combined. Around 30% of the influent BOD leaves a primary facultative pond in the form of methane (Marais, 1970). This type of pond is designed generally for the treatment of weaker wastes and in sensitive locations where anaerobic ponds odor would be unacceptable. 3.2.1. BOD Removal The activity of further anaerobic oxidation and the aerobic conversion of effluent to carbon dioxide, water and new bacterial and algae cells can result in removal of 80% of the BOD5 of the effluent flowing into the facultative pond (which means an overall removal in the order of 95% over the two ponds). This removal, and the subsequent quality of the outflow, depends on:
Moreover, as a result of the algal-bacterial activities described in the previous section, a high proportion of the BOD that does not leave the pond as methane ends up as algal cells. Thus in secondary facultative ponds (and in the upper layers of primary facultative ponds) "sewage BOD" is converted into "algal BOD" and this has important implications for effluent quality requirements. This provides even better BOD quality of the effluent from a facultative ponds as most of the BOD contained (70 to 90%) will be "algal BOD". When a facultative pond is used as a primary treatment, BOD removal may be very efficient. Abis (2002) reported a BOD removal in a pilot-scale facultative ponds in the United Kingdom (surface loading 51-117 kg/ha d) to an average of 91% (between 67.5% and 98.6%). These values include the contribution of algae in the effluent. With the algal (and other) solids removed from the effluent, the average removal was 97.2% (with a range of 89.7-99.7%).
Faecal bacteria are mainly removed in facultative and especially maturation ponds whose size and number determine the numbers of faecal bacteria (usually modeled in terms of faecal coliforms) in the final effluent, although there is some removal in anaerobic ponds principally by sedimentation of solids-associated bacteria. The principal mechanisms for faecal bacterial removal in facultative and maturation ponds are now known to be:
Regarding viruses removal, Little is definitely known about the mechanisms of viral removal in WSP, but it is generally recognized that it occurs by adsorption on to settleable solids (including the pond algae) and consequent sedimentation. Some parasites can be removed as well. Protozoan cysts and helminth eggs are removed by sedimentation. Their settling velocities are quite high (for example, 3.4 x10-4 m/s in the case of Ascaris lumbricoides), and consequently most removal takes place in the anaerobic and facultative ponds. It has recently become possible to design WSP for helminth egg removal (Ayres et al., 1992).
Nitrogen In facultative and maturation ponds, ammonia is incorporated into new algal biomass. Eventually the algae become moribund and settle to the bottom of the pond; around 20% of the algal cell mass is non-biodegradable and the nitrogen associated with this fraction remains immobilized in the pond sediment. That associated with the biodegradable fraction eventually diffuses back into the pond liquid and is recycled back into algal cells to start the process again. At high pH, some of the ammonia will leave the pond by volatilization. Mara and Pearson (1986) point out that under certain conditions some algal species are able to adapt to and withstand concentrations of up to 50 mg/l. There is little evidence for nitrification (and hence denitrification, unless the wastewater is high in nitrates). The populations of nitrifying bacteria are very low in WSP due primarily to the absence of physical attachment sites in the aerobic zone, although inhibition by the pond algae may also occur. Total nitrogen removal in WSP systems can reach 80% or more, and ammonia removal can be as high as 95%. Phosphorus The efficiency of total phosphorus removal in WSP depends on how much leaves the pond water column and enters the pond sediments. This occurs due to sedimentation as organic P in the algal biomass and precipitation as inorganic P (principally as hydroxyapatite at pH levels above 9.5), compared to the quantity that returns through mineralization and resolubilization. As with nitrogen, the phosphorus associated with the non-biodegradable fraction of the algal cells remains in the sediments. Thus the best way of increasing phosphorus removal in WSP is to increase the number of maturation ponds, so that progressively more and more phosphorus becomes immobilized in the sediments. From a well functioning two-pond system, 70% mass removal of total phosphorus may be expected. Heavy Metals Polprasert and Charnpratheep (1989) and Kaplan et al. (1987) examined the fate of heavy metals in such ponds. Adsorption of metals was increased in attached-growth stabilization pond as compared to stabilization ponds without attached-growth. Kaplan et al. reports only a slight decrease in total metals concentration, however the particulate fraction was mostly solubilized. A study by Moshe (1972) showed that high concentrations of metal ions (Cd, Cu, Ni, Zn, and Cr) are toxic to Chlorella species, the most common species in stabilization ponds, and adversely affect pond efficiency. However, high pH (higher than 8) causes metal ions to precipitate and allows pond purification processes to occur normally.
Many techniques have been developed to remove the algae from effluents, these include rock filtration, grass plots, floating macrophytes and herbivorous fish. Also, the use of maturation ponds can reduce the algal concentration considerably provided the system is not overloaded. 4. Additional Technologies Used to Improve WSP Effluent The use of anaerobic and facultative ponds system, as the only wastewater treatment before final discharge, was proven to be satisfactory under different circumstances and for various agricultural and aquacultural effluent reuses (Mara 2001, Pearson et al 1996). However, when some of the effluent quality limits are not satisfied, choosing a supplementary (or even alternative technology) in order to improve the effluent quality will be a serious option. The choice of adding new agents to the existing anaerobic and facultative ponds or choosing more advanced SWP treatment systems should be taken in the light of the following factors:
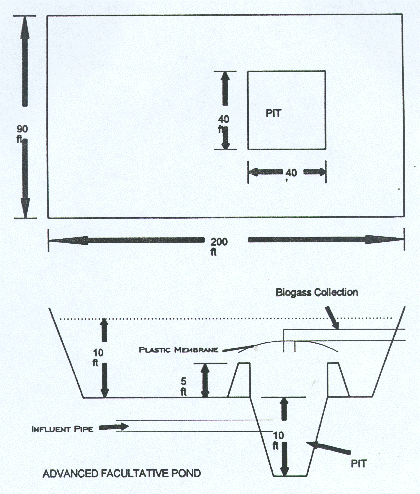
One possible solution to benefit from the advantages of both anaerobic and aerobic ponds and suppress their disadvantages is to integrate the best functions of each pond type into a single pond to allow the symbiotic relationships of related microorganisms to proceed unrestrained (Fig. 5). The advanced facultative pond is deep to promote sedimentation of wastewater solids and anaerobic decomposition of methane. Its most attractive feature is its high capability of wastewater total suspended solids (TSS) removal, in addition to BOD removal. The pond is designed so that its surface remains aerobic, thus reducing potential odor problem. Biogas may be collected using submerged gas canopy and potentially used for energy production. Until these integrated systems have been fully developed, most designers will continue to rely upon the traditional stabilization pond treatment systems.
Fig. 5 Integrated (Advanced) Facultative Pond.
Aeration introduces oxygen to effluent standing in a facultative pond, so that bacteria can effectively convert the organic solids to carbon dioxide, water and bacteria biomass. Mechanically aerated ponds generate turbulence to mix all the effluent in the pond and introduce oxygen through equipment that either
Aerator numbers and configuration are selected to perform the amount of oxygen generation needed. This technology can significantly reduce the nutrient, ammonia, odor, and BOD level in the resulted effluent. However, cost of the aerators including installation, operation and maintenance shall be taken into account in order to assess the feasibility of using such equipment (this basically varies from one project to another).
This involves of using microorganisms to turn the complex organic solids less complex compounds. The end products of anaerobic digestion are biogas (mix of methane and carbon dioxide) and a stabilized treated liquid. The biogas can be collected and used as an alternative energy source, but a storage space is required to fulfill this operation. This procedure reduces BOD but not the nutrient. In addition, Anaerobic digestion adds more complexity, equipment and cost to the overall effluent treatment system. A facultative pond treatment would still be required to improve the quality of the effluent.
Several kinds of additives are available to control odors and break down crusting and organic matter. The main ones are the followings:
In the pond modified by Zhao and Wang (1996), attached-growth media (AGM) or so-called artificial fibrous carriers were installed. This type of media consists of fine strings of polyvinyl acetate, with specific surface area of 1,236 m2/m3 and cost only US$ 5/m3. A pilot-scale investigation has been conducted by them, using three ponds with working dimensions of 4.0 m in depth, 1.2 m in width and 1.1 m in depth. This study has confirmed that the incorporation of AGM enhanced the performance of conventional WSPs by formation of a great number of small stable ecological systems around AGM, being abundant in bio-species from bacteria and algae to protozoa, increasing the biomass concentration, improving the biological distribution. Better removal efficiencies of COD (75.6%), BOD (90.2%) and NH4-N (68.5%) had been achieved in the WSPs with AGM than in the conventional WSPs, although the total retention time had been shortened to 7.5 days. Although capital investment in the system may increase, the system holds the potential to reduce retention times and decrease spatial requirements of the WSP technology (Yu, et al., 1997). [Top]
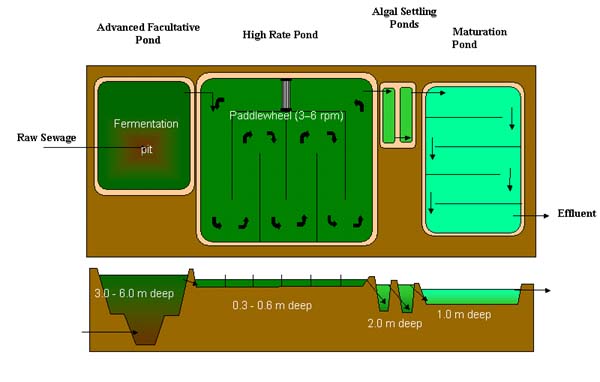
Developed by Professor William J. Oswald and his co-workers at the University of California, Berkeley over the past four decades wastewater treatment and algae production systems called Advanced Integrated Wastewater Pond Systems (AIWPS) are potentially feasible for application in the developing world (Oswald, 1990). Although AIWPS may appear to be an adapted traditional pond system, each AIWPS facility is uniquely designed and incorporates a series of low-cost ponds or earthwork reactors. Depending on specific effluent characteristics, regulatory requirements, human resources, and local climatic conditions, a typical AIWPS facility consists of at least four ponds in series (Fig. 6):
AIWPS facilities are designed to minimize the accumulation of sludge and to maximize the production of oxygen through algal photosynthesis. Algal biomass is produced and can be used as a nitrogen-rich fertilizer, or as protein-rich animal or fish feed (for further cultivation of high protein foodstuffs), modern medicine and even cosmetics for the idle. They are cost-effective, require little maintenance and have generally performed well in terms of BOD5 and solids removal. Moreover, AIWPS require similar land area to conventional lagoons, virtually eliminate sludge disposal, produce less odor, and may be adapted to energy (methane) recovery. However, AIWPS cost about $15,000 to set up, and $100 a year to power the paddle wheel and the algal settling pond needs to be desludged once to twice a year. In addition, note that this type of technology is not energy cost free.
Fig. 6 AIWSP system (adapted from NWA website).
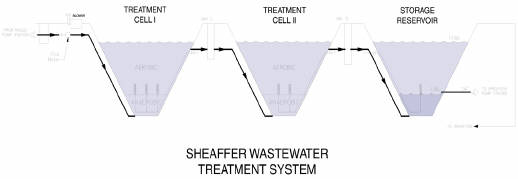
Sheaffer International markets a variation of the AIWPS described in the preceding section. The Sheaffer system is described as a Modular Reclamation and Reuse System producing no sludge, no odor, and enabling 100% recovery of nutrient rich water for irrigation. The system is comprised of a deep aerated treatment cell, a storage cell, and three moving parts, described as a grinder pump, a compressor/blower, and an irrigation system (Sheaffer International LTD., 1998). The first stage of the process uses the grinder pump to reduce sewage solids influent and injects it to an anaerobic zone at the bottom of the treatment cell where it undergoes anaerobic reduction for a 14- to 30-day period. This zone acts as a mesophilic reactor. Solids settle out of the anaerobic zone to the base of the deep cell, and are stored for a time period of 20 to 30 years. The second stage of the process, the compressor/blower, injects air into the treatment cell just above the anaerobic zone to create aerobic conditions at the surface level of the cell. The cells are designed to provide 14- to 36-day treatment and further reductions of organic materials (Sheaffer International LTD., 1998). Solid components are broken down into simple organic acids, methane carbon dioxide, sulphide, ammonia, inorganic compounds, and water. The nitrogen, phosphorus, and potassium are dissolved and remain in solution for use in agricultural irrigation.
Fig. 7 SMRRS (Sheaffer International LTD. 1998).
A number of facultative ponds have been designed, or more commonly retrofitted, with surface aerators to boost dissolved oxygen levels and/or to aid mixing. There is often confusion between these systems and what are typically called aerated lagoons. Unlike facultative ponds, aerated lagoons are designed to operate at high bacterial cell mass concentrations. These require a high power input for aeration and in some cases incorporate biomass return. They operate at much shorter hydraulic residence times and as a consequence of this, and their increased depth, do not develop significant algal populations. Aerated lagoons are essentially designed to work as a form of lowly loaded activated sludge. Mechanically supplied oxygen increases treatment efficiency and reduces land requirements. However, the high-cost power input is sufficient only for diffusing oxygen into the pond and not for mixing the contents. [Top]
Originally developed by Oswald at the University of California in the sixties, high-rate algal ponds have continued to be developed and implemented particularly in the United States. These systems are shallower than a facultative pond and operate at shorter hydraulic retention times. A paddlewheel is normally incorporated to drive the water around a "race-track" shaped pond. The oxygen production is reported to be significantly higher than typical facultative pond designs. The micro algae produced in these systems are also reported to have good settling properties (Green et al., 1996).
Waste stabilization ponds often have high concentrations of TSS in the effluent, which may or may not be desirable depending on the irrigation delivery method. Several polishing options are feasible to use in combination with WSPs to upgrade pond effluents, thereby increasing the options for effluent reuse. Middlebrooks (1995) suggests that many low-cost methods exist for polishing WSP effluent, which include intermittent sand filtration and rock filters. Rock filters, when used in conjunction with WSPs, have been shown to upgrade WSP effluent. Research at a pilot-scale rock filter demonstration conducted at the Assamra WSPs in Jordan showed that effluent content reductions could be reduced greatly. TSS and BOD were reduced by 60%, total faecal coliform count (TFCC) by a maximum of 94% and T-P by 46% at a loading rate of 0.33-0.044 kg/m3 of TSS (Saidam, Ramadan and Butler, 1995). If high levels of TSS are not an issue in an irrigation scheme and there is no risk of clogging irrigation equipment, high TSS may be advantageous as they will add organic matter to the soil matrix.
Maturation ponds (low-cost polishing ponds, which succeed the primary or secondary facultative pond) are primarily designed for tertiary treatment, i.e., the removal of pathogens, nutrients and possibly algae. They are very shallow (usually around 1 m depth, although Mara (1997) believes that at this reduced depth emergent plant growth and mosquito breeding problems can result) to allow light penetration to the bottom and aerobic conditions throughout the whole depth. The ponds follow a secondary treatment i.e., a facultative pond. The size and number of maturation ponds needed in series is determined by the required retention time to achieve a specified effluent pathogen concentration. In the absence of effluent limits for pathogens, maturation ponds act as a buffer for facultative pond failure and are useful for nutrient removal (Mara and Pearson, 1998). Mara (1997) notes that if an anaerobic and secondary facultative pond system is used, this will produce an effluent suitable for restricted irrigation. Therefore, additional maturation ponds will only be needed if a higher quality effluent is required. Another technology that may replace maturation ponds to improve WSP system performance is the use of constructed wetlands. Wetlands are areas which support the growth of a variety of plant species adapted to flooded conditions for part of, or the entire, year. The plants are densely spaced and, together with the shallow water, provide habitats for animal, bird and insect communities. Constructed wetland systems are designed to simulate and optimize filtering and biodegradation processes that occur in natural wetlands. They are a possible solution to improve the performance of pond systems, as they can "polish" wastewater effluent before its discharge to a waterway. During summer months, such a system may even result in zero discharge to waterways, due to evaporation and evapotranspiration of the water component from the wetland. [Top]
5. Siting of Ponds and Geotechnical Aspects When choosing a site to construct a pond system, an area should be selected where the water table is deep and the soil is heavy and impermeable. Silt or clay soils are ideal for pond foundations and construction. Building ponds over coarse sands, gravels, fractured rock or other materials, that will allow effluent to seep out of the pond or allow groundwater to enter in, should be avoided. No part of the system to be within 200 m (preferably 500 m) of any dwelling house. If possible, ponds should be sited downwind from dwellings, roads and other public places. The greater the distance from a potential complainant the better. Soil must be suitable for pond stability. Geotechnical aspects, if not taken into consideration, may cause the WSP system to malfunction. A geotechnical investigation of the site should be made during the design stage to ensure correct embankment design and to determine whether the soil is sufficiently permeable to require the pond to be lined. A stable and impermeable embankment core shall be formed, whether chosen from an available local or imported soil. After compaction, the soil should have a coefficient of permeability of 10-7 m/s (Mara and Pearson, 1998). The following geotechnical considerations should be taken when constructing the embankment:
The following are additional general considerations when siting a pond:
Wastewater treatment of only anaerobic and facultative ponds is widely considered as the most pragmatic option (at least as initial treatment). These two types, when used in series, are proven to be the most economical water treatment system with an effective performance. Basically, there are four approaches to wastewater stabilization pond design: loading rates, empirical design equations, reactor theory, and mechanistic modeling. Loading rates, as a design criterion, is a simple approach, widely used and recommended in most of the wastewater standard design handbooks worldwide. 6.1. Effluent Limits Effluent limits represent the maximum amount of pollutants allowed to discharge from wastewater to its final destination (waterway, reservoir for reuse, etc.). These limits vary from country to another due to geographical, climatic and socio-economical reasons. They vary as well with the character of the wastewater final destination. For example, the effluent quality of wastewater discharged to the ocean would be less stringent than the effluent quality of wastewater used for agriculture. Effluent limits characterize the required and accepted quality of the discharged wastewater. Hence, prior to design, these limits must be known (from local municipal effluent standards publications) since they will be used as the water quality design objectives. An example is the European Union quality requirements for WSP effluents being discharged into surface and coastal waters: Filtered BOD = 25 mg/l (non-algal BOD) Filtered COD = 125 mg/l (non-algal COD) Suspended solids = 150 mg/l Together with, for discharge into designated "sensitive areas subject to eutrophication": Total nitrogen = 15 mg/l Total phosphorus = 2 mg/l (Although, if the population served is > 100,000, these last two requirements are reduced to 10 and 1 mg/l, respectively) (Council of the European Communities, 1991a). Another example is from India. The general standards for the discharge of treated wastewaters into inland surface waters are given in the Environment Protection Rules (CPCB, 1996). The more important of these for WSP design are as follow: BOD 30 mg/l (non-filtered) Suspended solids 100 mg/l Total N 100 mg N/l Total ammonia 50 mg N/l Free ammonia 5 mg N/l Sulphide 2 mg/l pH 5.5 – 9.0 [Top]6.2. Design Parameters The four most important parameters for WSP design are:
6.3. Loading and Retention Time Any pond treatment system requires steady effluent flow to encourage the rapid and continuous growth of bacteria involved in the biological breakdown of effluent. It is essential that the daily loading into the ponds is kept to the design standards of the pond system. A very large load may flush out important bacteria, eventually leading to system failure. Variation in loads will alter the retention time. Any attempt to extend the time that effluent remains within the pond system will increase the amount of disease-causing microorganism die-off. The concentration of microorganisms within the effluent will be reduced and the effluent will be of higher quality before discharge into a waterway. 6.4. Loading Rates Design Approach This approach involves a "black box" type of design, where a ratio of a parameter such as population, flow or BOD is used in relation to the required volume or area of pond. This simplified approach to the process design of pond systems has been very commonly used throughout the world. For example, in the case of New Zealand, a figure of 84 kg BOD/ha.day (MWD, 1974), has been routinely used for facultative pond design regardless of the marked differences in environmental conditions throughout the country. 6.4.1. Anaerobic Ponds Design Anaerobic ponds can be satisfactorily designed, and without risk of odor nuisance, on the basis of volumetric BOD loading (lv, g/m3d), which is given by: lv = Li Q / Va where Li = influent BOD, mg/l (= g/m3 ) Q = flow, m3/d Va = anaerobic pond volume, m3
Table 3. Design values for anaerobic ponds (Mara and Pearson 1996).
l v can even reach 400 g/m3 d, but in this table the upper limit of 350 is used to provide an adequate margin of safety with respect to odor. Note that permissible volumetric BOD loadings lv should not be less than 100 g/m3 d in order to maintain anaerobic conditions. This is appropriate for normal domestic or municipal wastewaters, which contain less than 300 mg/l SO4-.
qa = Va / Q (minimum 1 day should be used, if calculations gives < 1 day, a value of 1 day should be used and the new value of Va should be recalculated).
Ls = 10 Li Q / Af where Af = facultative pond area, m2
Ls= 20T - 120 However, more appropriate global design equation was given by Mara (1987): ls = 350 (1.107 - 0.002T)T-25
qf = Af D / Qm where D = pond depth, m (see section 3.2) Qm = mean flow, m3/day The mean flow is the mean of the influent and effluent flows (Qi and Qe), the latter being the former less net evaporation and seepage. Thus: qf = Af D / [1/2 (Qi + Qe)] If seepage is negligible, Qe is given by: Qe = Qi – 0.001 e Af where = net evaporation rate, mm/day. Thus: qf = 2 AfD / (2 Qi – 0.001 e Af)
Pano and Middlebrooks (1982) present equations for ammonical nitrogen (NH3 + NH+4) removal in individual facultative (and maturation) ponds. Their equation for temperatures below 20 oC is:
Ce = Ci / {1 + [(A / Q) (0.0038 + 0.000134T) exp ((1.041 + 0.044T)(pH - 6.6))]} and for temperatures above 20 oC: Ce = Ci / {1 + [5.035 × 10-3 (A / Q)] [exp(1.540 × (pH - 6.6))]} where Ce = ammoniacal nitrogen concentration in pond effluent, mg N/l Ci = ammoniacal nitrogen concentration in pond influent, mg N/l A = pond area, m2 Q = influent flow rate, m3 /d Reed (1985) presents an equation for the removal of total nitrogen in individual facultative (and maturation) ponds: Ce = Ci exp{-[0.0064 (1.039)T-20] [q + 60.6 (pH - 6.6)]} where Ce = total nitrogen concentration in pond effluent, mg N/l Ci = total nitrogen concentration in pond influent, mg N/l T = temperature, oC (range: 1-28oC) q = retention time, d (range 5- 231 d) The pH value used in the previous equations may be estimated from: pH = 7.3 exp(0.0005 Ai) where Ai= influent alkalinity, mg CaCO3/l The equations shown can be applied sequentially to individual facultative and maturation ponds in the series, so that concentrations
in the effluent can be determined. Phosphorus There are no design equations for phosphorus removal in WSP. Huang and Gloyna (1984) indicate that, if BOD removal in a pond
system is 90 percent, the removal of total phosphorus is around 45 percent. Effluent total P is around two thirds inorganic and one-third organic. 6.5. Hydraulic Balance To maintain the liquid level in the ponds, the inflow must be, at least, greater than net evaporation and seepage at all times. Thus: Qi = 0.001 A (e + s) where Qi = inflow to first pond, m3/d A = total area of pond series, m2 e = net evaporation (i.e. evaporation less rainfall), mm/d s = seepage, mm/d [Top]6.6. Process Design for Wastewater Discharged in a Waterway
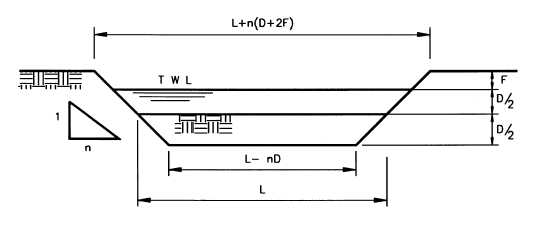
F= (log10A)1/2 - 1 where F= freeboard (m) and A= pond area (m2) at top water level. Va = [(L W) + (L - 2sD) (W - 2sD) + 4(L - sD) (W - sD)] [D / 6] where Va = anaerobic pond volume, m3 L = pond length at TWL, m W = pond width at TWL, m s = horizontal slope factor (i.e. a slope of 1 in s) D = pond liquid depth, m With the substitution of L as nW, based on a length to breadth ratio of n to 1, the equation becomes a simple quadratic in W
Fig. 8 Geometry of pond (Mara and Pearson, 1998). Approximation of the land areas required per caput for anaerobic and facultative ponds can be calculated. This would be very beneficial, especially during the planning phase, when land availability and price are to be considered as a key factor for final decision on the type of wastewater treatment chosen. 6.8.1. Anaerobic Ponds The equation presented in section 4.5.1 can be rewritten as: Aa = Li Q / D lv Where Aa = anaerobic pond area, m2/caput Li Q = quantity of BOD, g/caput day D = anaerobic pond depth, m lv as described above
6.8.2. Facultative Ponds The equation presented on 4.5.2 can be rewritten as: Af = 10 Li Q/ ls where Af = facultative pond area, m2/caput Li Q = quantity of BOD, g/caput day ls as described above Note that total area calculated (Aa + Af) shall be multiplied by a factor of 1.25-1.5 (i.e., additional 25% to 50% land) to take into account the overall land area required for pond operation and maintenance. 1.25 factor is suitable for large systems while 1.5 factor is more suitable for small systems (Mara, 1998). When maturation ponds are required the additional land area required for building and maintaining these ponds shall be added. [Top]6.9. WSP Hydraulics Considerations Finney and Middlebrooks (1980) stated that consistent prediction of pond performance by any design method without accurate projections of hydraulic residence time is impossible. Shilton (2001) presented an extensive study on the hydraulics of stabilization ponds. Twenty experimental configurations were tested in the laboratory and ten of these experimental cases were mathematically modeled and had good agreement with the experimental work. Shilton and Harrison (2003) then introduced broad and informative guidelines for hydraulic design of WSP to "help fill the knowledge gap in the pond hydraulics area". Although engineering judgment is always required, and the current understanding of ponds hydraulics is still limited, the following observations were proven to be useful for the purpose of improving WSP hydraulics, and consequently ameliorating WSP design, performance and efficiency:
Filtered BOD < 25 mg/l TTS < 150 mg/l Nematode egg < 1 /l Faecal coliform count < 1000 per 100 ml (for unrestricted irrigation only). Abis, Karen L. (2002). The performance of facultative waste stabilization ponds in the United Kingdom. Ph.D. thesis, U.K. University of Leeds. Arridge, H., Oragui, J.I., Pearson, H.W., Mara, D.D. and Silva, S.A. (1995). Vibrio Cholerae 01 and Sallmonellae removal compared with the die-off of faecal indicator organisms in waste stabilization ponds in northeast Brazil. Water Science Technology, 31 (12), pp.249-256. Arthur J.P. (1983). Notes on the design and operation of waste stabilization ponds in warm climates of developing countries. Urban Development Technical Paper No. 6. World Bank, Washington DC. 106 p. Ayres, R.M., Alabaster, G.P., Mara, D.D. and Lee, D.L. (1992). A design equation for human intestinal nematode egg removal in waste stabilization ponds. Water Research, 26 (6), 863-865. Bartone, C. R. (1991). International perspective on water resources management and wastewater reuse - appropriate technologies. Water, Science & Technology, 23: 2039-2047. Boutin, P., Vachon, A. and Racault, Y. (1987). Waste stabilization ponds in France: an overall view. Water Science and Technology, 19 (12), pp.25-31. Bucksteeg, K. (1987). German experiences with sewage treatment ponds. Water science and Technology, 19 (12), pp.17-23. Council of the European Communities (1991). Council Directive of 21 May 1991 concerning urban wastewater treatment (91/271/EEC). Official Journal of the European Communities, L135/40 (30 May). CPCB (1996). Pollution Control Acts, Rules and Notifications Issued Thereunder, 4th edition. New Delhi: Central Pollution Control Board. Droste, R.L. (1997). Theory and Practice of Water and wastewater Treatment. John Wiley and Sons. New York, USA. EPA (1983). Design Manual: Municipal Wastewater stabilization Ponds. Report No. EPA-625/1-83-015. Cincinnati: Environmental Protection Agency, Center for Environmental Research information. Finney, B. and Middlebrooks, E. (1980). Facultative waste stabilization pond design. Journal of the Water Pollution Control Federation, 52(1): 134-147. Gambrill M.P., Mara D.D., Eccles C.R. and Baghaei-Yazdi N. Microcomputer-aided design of waste stabilization ponds in tourist areas of Mediterranean Europe. Public Health Engineer. 14(2): 39-41. Glonya, E.F. and Espino, E. (1969). Sulfide production in waste stabilization ponds. Journal of the Sanitary Engineering Division, American Society of Civil Engineers, 95(SA3), pp.607-628. Green, F., Bernstone, L., Lundquist, T. and Oswald, W. (1996). Advanced integrated wastewater pond systems for nitrogen removal. Water Science and Technology. 33(7):207-217. Kaplan, Drora, Abeliovich, Aharon et Ben-Yaakov, Sam. (1987). The Fate of Heavy Metals in Wastewater Stabilization Ponds. Water Research, Vol. 21, No. 10, 1189-1194. Knorr, A.E. and Torrella, F. (1995). Microbiological performance and Salmonella dynamics in a wastewater depuration pond system of southeastern Spain. Water Science Technology, 31 (12), pp.239-248. Mara D.D. (1976) Sewage Treatment in Hot Climates. John Wiley, London. Mara, D.D. (2001), Low-cost, high performance wastewater treatment and reuse for public health and environmental protection in the 21st century. 10th Annual CWWA conference on Innovation Technologies in the Water and Wastewater Industries for the 21st century, Grand Cayman October 2001. Mara, D. D., Alabaster, G.P., Pearson, H.W., Mills S.W. (1992). Waste Stabilisation Ponds - A Design Manual for Eastern Africa. Leeds: Lagoon Technology International Ltd. Mara, D. D. and Pearson, H. (1998). Design Manual for Waste Stabilization Ponds in Mediterranean Countries, Leeds: Lagoon Technology International Ltd. Mara, D. D. and Pearson, H. (1986). Artificial Freshwater Environment: Waste Stabilisation Ponds. In: Biotechnology (Rehm and Reeds, eds.). VCH Verlagsgesellschaft, Weinheim, Germany. Mara, D.D., Pearson, H.W., Oragui, J.I, Arridge, H. and Silva, S.A.(2001). Development of a new approach to waste stabilization pond design. School of Civil Engineering, University of Leeds, Leeds, England. Marais, G.v.R. (1970). Dynamic behaviour of oxidation ponds. In Proceedings of the Second International Symposium on Waste Treatment Lagoons (ed. R.E. McKinney), pp. 15-46. Laurence, KS: University of Kansas. McGarry M.G. and Pescod M.B. (1970) Stabilization pond design criteria for tropical Asia. Proc. 2nd Int. on Waste Treatment Lagoons, Kansas City, pp.114-132. Meiring P.G., Drews R.J., van Eck H. and Stander G.J. (1968) A guide to the use of pond systems in South Africa for the purification of raw and partially treated sewage. CSIR Special Report WAT34. National Institute for Water Research, Pretoria. Middlebrooks, E. J. (1995). Upgrading pond effluents: An overview. Water, Science & Technology, 31 (12): 353-368. Moshe, Meir. (1972). Effect of Industrial Wastes on Oxidation Pond Performance. Water Research, Vol. 6, 1165-1171. Oragui, J.I, Arridge, H., Mara, D.D., Pearson, H.W. and Silva, S.A. (1995). Rotavirus removal in experimental waste stabilization pond system with different geometries and configurations. Water Science Technology, 31 (12), pp.285-290. Oswald, W. J. (1990). Advanced integrated wastewater pond systems. Proceedings of the ASCE convention: Supplying water and saving the environment for six billion people. San Franciso, CA., 5-8 Nov, 1990, pp: 73-80. Pano, A. and Middlebrooks, E.J. (1982). Ammonia nitrogen removal in facultative wastewater stabilization ponds. Journal of the Water Pollution Control Federation, 54(4), 344-351. Pearson, H. W., Mara, D. D. and Arridge H.M. (1995). The effluence of pong geometry and configuration of facultative and maturation waste stabilization pond performance and efficiency. Water Science Technology, Vol. 31 No.12, pp.129-139. Pearson, H. W., Mara, D. D., Crawley L.R., Arridge H.M. and Silva S.A. (1996). The performance of an innovative tropical experimental waste stabilization pond system operation at high organic loadings. Water Science Technology, Vol. 33 No.7, pp.63-73. Pescod M.B. and Mara D.D. (1988) Design, operation and maintenance of wastewater stabilization ponds. Ch. p, Treatment and Use of Sewage Effluent for Irrigation. M.B. Pescod and A. Arar (eds). Butterworths, Sevenoaks, Kent. Polprasert, C. and Charnpratheep, K. (1989). Heavy Metal Removal in Attached-Growth Waste Stabilization Ponds. Water Research, Vol. 23, No. 5, 625-631. Reed, S.C. (1985). Nitrogen removal in wastewater stabilization ponds. Journal of the Water Pollution Control Federation, 57 (1), 39-45. Rinzima, A. (1988). Anaerobic treatment of wastewater with high concentrations of lipids and sulphate. Ph.D. thesis, Wageningen Agricultural University, Wageningen, The Netherlands. Saidam, M., Ramadan, S. & Butler, D. (1995). Upgrading waste stabilization pond effluent by rock filter. Water Science & Technology, 31(12): 369-378. Shaw, V.A. (1962).An assessment of the probable influence of evaporation and seepage on oxidation pond design and construction. Journal of the Institute of Sewage Purification, (4), 359-365. Sheaffer International LTD. (1998). An idea whose time has come. Sheaffer International LTD., Promotional literature. Shilton Andy (2001). Studies Into the Hydraulics of Waste Stabilization ponds. Ph.D. Thesis. Massey University, Palmerston North, New Zealand. Shilton A. and Harrison J. (2003). Guidelines for the Hydraulic Design of the Waste Stabilization ponds. Institute of Technology and Engineering, Massey University, Palmerston North, New Zealand. Shuval H.I., Adin A., Fattal B., Rawitz E. and Yekutiel P. (1986) Wastewater irrigation in developing countries: health effects and technical solutions. Technical Paper No. 51. World Bank, Washington DC. Soares, J., Silva, S.A., Oliveira, R., Araujo, A.L.C., Mara, D.D. and Pearson, H.W. (1996). Ammonia removal in a pilot-scale WSP complex in northeast Brazil. Water Science Technology, 33 (7), pp.165-171. Uddin, M. S. (1970). Anaerobic Pond Treatment of Tapioca Starch Waste, Master Thesis. Bangkok: Asian Institute of Technology. Tchobanoglous, G. and Schroeder, E. (1985). Water Quality Characteristics, Modeling, Modification. Addison-Wesley; Reading, Massachusetts, USA. Van Haandel, A.C., Lettinga, G. (1994). Anaerobic Sewage Treatment: A Practical Guide for Regions with a Hot Climate. John Wiley & Sons. Yu, H., Tay, J. & Wilson, F. (1997). A sustainable municipal wastewater treatment process for tropical and subtropical regions in developing countries. Water, Science & Technology, 35 (9): 191-198. Zendher, A.J., Ingvorsen, K. and Marti, T. (1982). Microbiology of methanogen bacteria. In: Anaerobic Digestion. Elsevier, Amsterdam, The Netherlands. Zhao, Q. & Wang, B.(1996). Evaluation on a pilot-scale attached-growth pond system treating domestic wastewater. Water Resource, 30: 242-245. [Top] |
| Home | Virgin Globe | CEE SDSU | Prof. V.M. Ponce | Contact Us |
 ;
;