|
THAT IS THE QUESTION |
♦ Irrigation and salinity: A pervasive problem ♦
The oceans cover about 70% of the Earth's
surface (Fig. 1).1
Throughout geologic time, the oceans have been the natural sink for all solid materials
transported by surface runoff.
The fact that the oceans contain 3.5% of dissolved solids, or 35,000 parts per million (ppm), and that 86% of these solids are ions of
sodium (Na+) and chloride
(Cl-) can mean only one thing:
sodium and chloride, significantly among other ions, have been accumulating in ocean waters for several hundred million
years.2
Marine life is responsible for the precipitation of calcium and other ions, but not sodium and chloride.
In addition, sodium chloride has a high solubility, as compared to most other salts.
Sodium chloride (common salt) is a unique compound. Its supply exceeds the demand almost everywhere and therefore, it has a natural
tendency to accumulate.
Its components, sodium and chloride, can be used to produce acids [hydrochloric acid] and bases [sodium hydroxide]
for industrial use, but there is a lot more sodium
chloride in nature than we could possibly ever use. Thus, sodium chloride is a waste product of the biosphere. Through millennia,
it has been carried off, together with other surplus materials, into the oceans by streams and rivers.
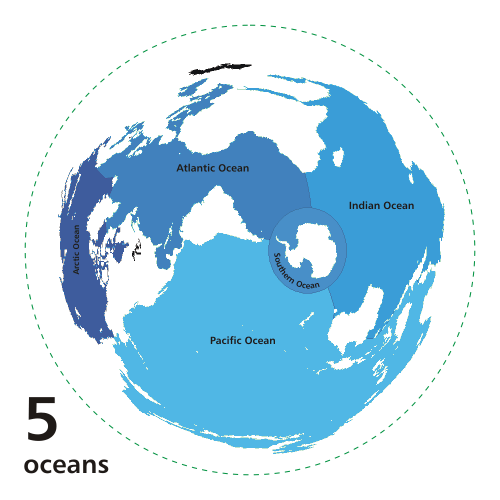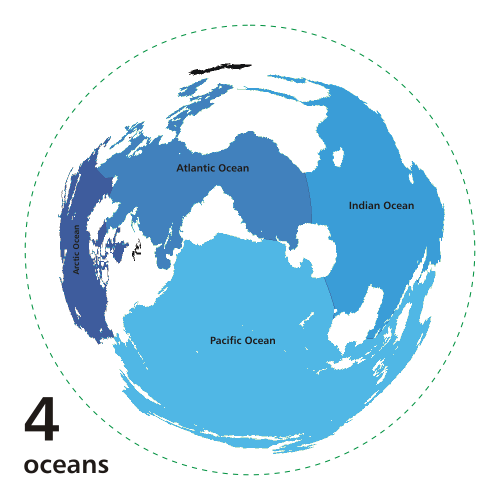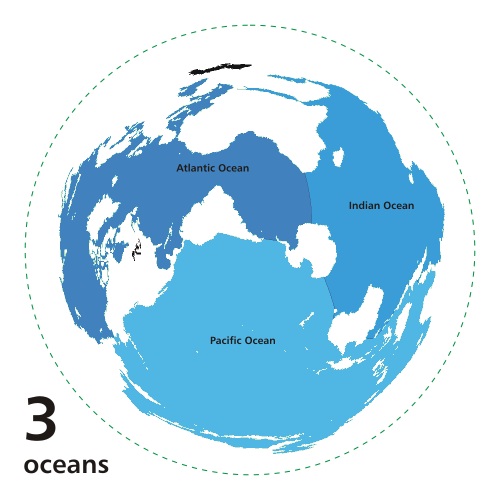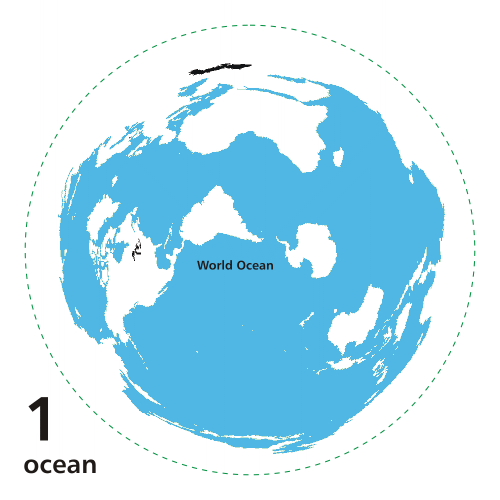
Fig. 1 The World's oceans
(Source: Wikipedia)
Salts are always present in the rocks; therefore, we may assume that they have been produced since the beginning of time.
If for whatever reason, salts are not transported to the oceans, they remain on the land and accumulate in the form of saline deposits, saline lakes, or saline terrestrial ecosystems, of which there are several good examples (Utah's Salt Lake is one).
The presence of salts sets the saline lake/ecosystem apart from others, because a saline environment can only support certain types of species.
Therefore, saline environments are uncommon in the natural terrestrial world.
The reason for the presence of salts in terrestrial and lacustrine ecosystems, other than at background levels, has to do with the local geology; specifically, geomorphology, tectonism, and relative continental location.
Typically, uplift and subsidence determine whether a drainage basin is exorheic, with free drainage, or endorheic, without free drainage.
An endorheic system has a tendency to accumulate salt over and above the typical background levels.
In geologic time, the waters can become brackish, saline, or brine. An example is Poopo Lake, in southwestern Bolivia.
The salinity of Poopo Lake varies between brackish and saline (15,000 to 30,000 ppm) at its northern end, near the water source, to brine (105,000 to 125,000 ppm) at its southern end.3
All terrestrial saline ecosystems have in common some type of impaired drainage; conversely, impaired drainage typically leads to a saline environment.
The essential purpose of rivers is to carry the solids, with salts being the most important solids, to the ocean.
Thus, evaporating runoff through additional evapotranspiration [irrigation] creates a problem of salt disposal.
In the absence of runoff to carry the salts, they would have to be mechanically transported to their ultimate destination, the oceans.
Therefore, there is a price to pay for the increased productivity of irrigation: How to dispose of the salts that are left behind.4
The problem is compounded by the fact that while a rainfed agricultural system produces one kind of salt, natural new, irrigated agriculture produces all four possible combinations of natural/artificial and new/old salts.
Thus, the problem of salt production and disposal in irrigated agriculture is much more serious than in its rainfed counterpart.
The pervasiveness of irrigation in society implies that excess salinity is also pervasive.
For this reason, many professionals have dedicated their lives to the management of salinity.5
Yet, there is one truism: Unless the excess salts are disposed off properly, ideally in the ocean, sustainability is not possible.
In the absence of effective ocean disposal, the salts accumulate in the environment, and sooner or later the terrestrial ecosystems degrade accordingly.
♦ Case Study: The San Joaquin Valley, California ♦
California is facing a salinity management problem that is sure to test the survivability of its agricultural industry in the medium term.
The San Joaquin valley has natural drainage problems, while at the same time it has an extensive system of irrigated agriculture (Fig. 2).
As explained earlier, these two do not mix very well.
Since 1995, the U.S. Bureau of Reclamation has focused on solving the problem, and it now appears that the "economic solution" of choice is not to drain, but rather to recycle the salts for a while and retire a fraction of the lands in the hope of mitigating the problem (U.S. Bureau of Reclamation, 2007).
Yet this solution is not only unsustainable [because the salts will continue to increase], but self-defeating as well, because it entails the retirement of lands, essentially amounting to cutting one's legs off.
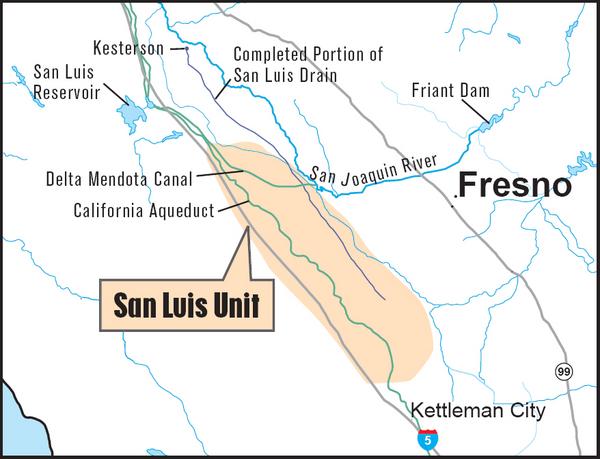
Fig. 2 Drainage-affected lands in the San Joaquin Valley of California
(Source: U.S. Bureau of Reclamation).
A sustainable solution is represented by ocean disposal; however, this solution appears to be out of favor because of perceived environmental impacts (State Water Resources Control Board, 2005).
A properly designed ocean disposal system would be more effective in the long run, and cost less, socially and economically, than the land retirement option.
Yet there are those who argue that ocean disposal will pollute the ocean beyond its capacity to recover.
To assuage worries, the brines could be treated before disposal.
Or, they could be dumped far enough into the ocean to minimize possible negative impacts to coastal resources.
One thing is certain: A relatively small brine discharge, estimated at 300 cubic feet per second (8.5 cubic meters per second), could not affect the vast oceans, which measure about 1,300,000,000 cubic kilometers.
For many years, the argument has been: "Dilution is the solution to pollution."
In a terrestrial water body, this statement usually requires some qualification. However, ocean dilution is the best possible type of dilution, because of the sheer enormity of the receiving waters.
For instance, the calculated residence time6 in the ocean of a discharge of 8.5 cubic meters per second is 4,850,000,000 years, which is somewhat longer than the age of the Earth.7
One successful example of ocean disposal is the Santa Ana Regional Interceptor (SARI) line, located in Riverside and Orange counties, California.
This project disposes of a mix of desalter concentrate and industrial wastewaters through a brine line, with final destination at a 5-mile long 200-feet deep ocean outfall in Huntington Beach.8
The technology of ocean disposal exists, and limited available experience indicates that it is not injurious.
The alternative, in-valley accumulation, is simply not sustainable.
Sustainability in salinity management will require a clear watershed vision and a healthy dose of political will.
In the present context, the only sustainable choice is to drain.
