|
RIVER BASIN SALINITY |
♦ Fact No. 1 ♦
Rivers always carry a certain amount of dissolved solids, among them, notably, salt ions.
The amount of dissolved solids varies widely, from lower than 100 ppm for some headwater streams, to more than 1,500 ppm for rivers heavily laden with salts.
For instance, the salinity of the Colorado River at Imperial Dam, California, is about 800 ppm.
♦ Fact No. 2 ♦
Dissolved solids in streams and rivers originate in the parent rocks.
Weathering and bioturbation are the processes by which the solids are released from the rocks and soils,
leaving the lithosphere through runoff and entering
the hydrosphere.

|
| Bioturbation |
♦ Fact No. 3 ♦
Several types of dissolved salt cations (positive) and anions (negative) enter the hydrosphere through runoff.
The salt cations are four: (1) sodium, (2) calcium, (3) potassium, and (4) magnesium. Typical salt anions are: (1) chloride, (2) sulfate, and (3) carbonate.
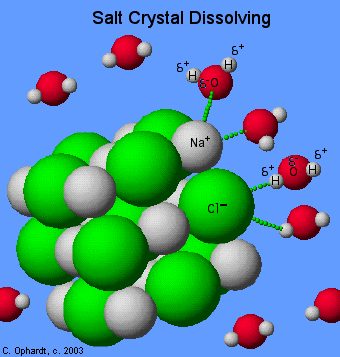
|
| Sodium chloride |
♦ Fact No. 4 ♦
Potassium and magnesium are mostly used by the biosphere, while sodium and calcium are not used by the biosphere
in the amounts in which they are present.
Potassium and magnesium are the good salts, needed by the biosphere.
Sodium and calcium are the bad salts, wasted by the biosphere.
♦ Fact No. 5 ♦
Evaporation and evapotranspiration leave the waste salts behind, concentrating them in the remaining runoff.
The amount of total dissolved solids (TDS) in a stream or river increases in the downstream direction.
♦ Fact No. 6 ♦
Headwater streams have a smaller concentration of dissolved solids than lowland rivers.
Under pristine conditions, the increase in TDS in the downstream direction is a natural characteristic of streams and rivers.
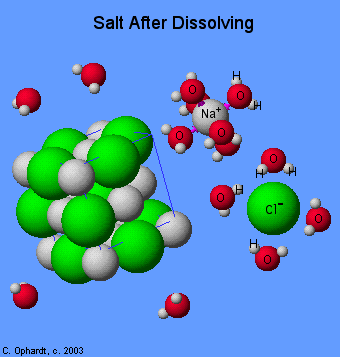
|
|
| Dissolution of sodium chloride in water. |
♦ Fact No. 7 ♦
Irrigation, a consumptive use of water, results in additional evapotranspiration, increasing the concentration of total dissolved solids in runoff waters (the hydrosphere).
Every iota of water used consumptively to grow crops leaves the salts behind and creates a problem of disposal.
♦ Fact No. 8 ♦
Storage reservoirs produce additional evaporation, increasing the concentration of total dissolved solids in the water body and,
therefore, in the hydrosphere.
Arid regions evaporate water at rates much higher than humid regions. Thus, arid regions
concentrate salts faster than humid regions.
♦ Fact No. 9 ♦
Rivers of developed basins have higher concentrations of TDS than rivers of pristine basins.
The additional salts to be mobilized increase the TDS concentration, unless they are routed to and collected in evaporation ponds.
♦ Fact No. 10 ♦
Evaporation ponds or basins take the extra salts away from the runoff (hydrosphere) and deposit them in the soils (lithosphere).
An evaporation pond or basin reduces or eliminates regional surface water anthropogenic salinity increases at the expense of local soil/land salinity increases.

|
| Evaporation pond in Tulare Lake basin, California. |
♦ Fact No. 11 ♦
An aquifer lying below an evaporation basin can become contaminated with salts percolating through the vadose zone,
which lies between the land surface and the groundwater table.
A properly designed liner may reduce this effect, but liners have a finite life.
♦ Fact No. 12 ♦
Aquifers flow from zones of higher potential to zones of lower potential in a direction that resembles and/or closely parallels the land surface.
The great majority of groundwater eventually makes it to the surface waters through baseflow.
On a global basis, only a very small fraction of groundwater (less than 2%) flows directly into the ocean through deep percolation,
avoiding the surface waters altogether.
♦ Fact No. 13 ♦
The more intense a river basin is developed for irrigation, the more the quantity of
salts that need to be handled or disposed, either through salt-laden runoff and streamflow (a regional impact),
or by storage in evaporation ponds (a local impact).
The choice is either: (1) to pollute the river, or (2) to first pollute the land, and then, eventually, through baseflow, the river. A third choice, to collect the salt and cart it to the ocean,
is usually prohibitively expensive.